Narrative of How This Happened to this Patient, by the patient
This aggressive surgery was on March 19, 2011.
I was taken to the emergency room following a car accident. I was alert and able to speak. I signed consent to be admitted to the emergency room.
While waiting in the ER laying on a gurney, I was sedated via injection to "keep me relaxed" as the UCLA nurse stated till it was my turn to be seen. My medical records indicate I was being injected every 15 minutes apparently till I was unable to respond.
I was taken to the OR without my consent. My records indicate the spine center residents tried to obtain my consent, but could not.
The UCLA Spine Resident working under UCLA Spine Center director Dr Langston Holly hand wrote "Unable to gain patient's consent...then falsely wrote, "Obtained verbal consent."
Dr Holly and his residents put me thru an 11 hour anterior and posterior cervical spine fusion with non-FDA approved devices. The Medtronic sales representative Brad Schaefer brought the devices to the OR and was present throughout the entire procedures.
In the last four + years, I’ve obtained second opinions from spine surgeons whom have told me I did not need the posterior spinal surgery.
I was also told that Medtronic did not have FDA approval for this instrumentation to be placed in the back of my cervical spine. UCLA operated on me without any signatures nor my consent whatsoever: an anterior and posterior cervical fusion with RECALLED non-FDA approved Medtronic devices. Neither Medtronic, nor Dr. Langston Holly nor anyone at UCLA ever "disclosed" to me that Medtronic was paying Royalties and in a POD (Physician Owned Distribution) with Dr Holly to implant, and teach the residents to implant, the Medtronic Vertex Max Titanium rods, screws and bar system in my spine.
I discovered today March 3, 2017, that Medtronic Vertex Max was RECALLED several times for issues with "loosening of screws" on the FDA.gov website. The RECALLS are found on the FDA.gov website.
The 1st recall was in 2010, then the years 2012, 2013, 2014.
Therefore, Medtronic knew during my 2011 surgery that their device was Recalled and Defective the year before in 2010 and still had Dr Holly place it in me in 2011.
Isn't this Punitive and Unconscionable? How can Medtronic knowingly do this? This is Fraud and Intentional Concealment.
Furthermore, it continued to be Recalled and Defective every year thereafter for the next 4 years. I never received any Notice of Recall.
Dr Holly could not resist the Undue Influence Medtronic had over him by Paying him Royalties to implant it in patients and advertise it on the internet.
Medtronic Intentionally Concealed the Recalls and Defect and continued to bill patients, Medicare, and all Insurance companies, becoming more and more profitable for its Fraud and Concealment while knowing patients were being catastrophically harmed and needing further spine fusion revision and reconstructive surgeries.
Medtronic had Brad Schaefer, a sales representative, showing Dr Holly and the residents how to implant the Medtronic Vertex Max. The Medtronic Rep was in my surgery room without my knowledge nor consent for these surgeries, this hardware nor his presence during the 10 hour surgery time.
I also found 6 "Adverse Events" of other patients who had loosening the screws as well, which like me, necessitated the removal of the hardware.
I was never informed that the devices would be used in manners not approved by the FDA, as Medtronic Vertex Max WAS NOT approved by the FDA. I contacted the Medtronic representative, Brad Schaefer within last 2 years as to why he provided Dr Holly the non-FDA approved Vertex Max, but he hung up on me. Dr Holly and UCLA did not disclose to me these Fraudulently arranged life altering surgery they performed on me without consent whatsoever. Dr Holly and UCLA internally concealed their Alterations of the screws drilled into my cervical spine from me and from the FDA. Dr Holly was supposed to notify the FDA of any complications from off-label use, as they are required to do by Law. Dr Holly intentionally concealed the surgical complications I have suffered.
Secondly, Dr. Langston Holly left the operating room three different times. My medical records show that he had clocked out and clocked back in leaving the residents to operate on me without supervision for almost 3 hours total.
After two weeks, I had 33 staples removed from that posterior 12 inch incision.
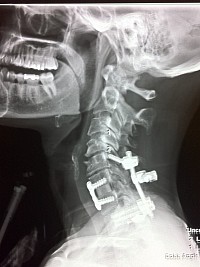
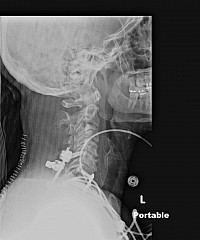
Within six weeks, my posterior spine began to split open. The internal suturing is visible in an X-Ray. It can be seen that the internal suturing did not suture the entire incision all the way up. The suturing was improper.
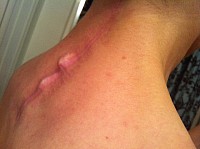
I suffered a severe Fascia Dehiscence of my entire trapezius triangle posterior spine; My spine had split open, my spinous process bones were protruding through my skin and the hardware and crosslink bar could be felt under my skin.
Thirdly, after 2 second opinion surgeons sent me for a CT scan, it was discovered that the screws were loose. It was also discovered that the left screw was inserted in my nerve exit (Neural Foramen), and not into the bone.
Fourth, my C6 disc was removed in totality, and an over-distracted rectangle spacer was placed. Dr. Langston Holly did not use the correct cervical spacer; A Cornerstone rectangle shaped block was used instead of a wedged spacer. Therefore, my cervical spine was fused without the necessary "lordosis"; Dr Holly fused me in a flat straight line.
A cervical osteotomy is required to revise the improper fusion position with more hardware; This means re-breaking the bones/ the spine fusion, re-breaking my cervical spine and fixating it into a proper alignment with more new hardware.
To date, I have already had a total 3 more surgeries. Two of them were to attempt to put my posterior anatomy back together by reattaching the 12 inch Fascia Dehiscence. The surgeries were not successful because of the of Dr Holly's negligence in delaying treatment. Dr Holly instead abandoned care for me, neglected to diagnose these tragic surgical problems, neglected to treat them and neglected to refer them to a specialist; This delay caused repair to be virtually difficult if not impossible because my posterior tissues had scarred down, retracted too far apart and foreshortened.
Furthermore, I'm still in need of the cervical osteotomy. I have bone spurs growing near and around my nerve exits and spinal cord too. I'm attempting to go into a fifth surgery to have the osteotomy revision spine fusion procedure and remove the bone spurs.
In this X-Ray, it can be seen that the internal suturing was not completed to the top of the incision, as was the external staples were. The internal sutures were stopped short, and the patient's posterior spine anatomy began to split open as a result of improper suturing.
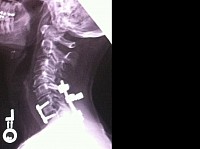
The fusion is straight; It is in a straight line, lacking proper plumb line and proper sagittal spine alignment. This was caused by the anterior spacer being a rectangle shape instead of a wedge triangle shape. Cervical lordosis is critical in proper spine fusion alignment. Instead, this patient was fused with cervical kyphosis - the opposite of what is correct.
The patient's spines process bones protruding through the skin. Crosslink bar felt when palpating the skin. Fascia and muscles splayed apart as a result of improper suturing and improper indicated use of the Vertex Max Reconstruction System crosslink bar and rods.