Post Surgery Photos
About Dr. Holly's FINANCIAL Relationship with Medtronic Conflict of Interest
Dr Langston Holly is paid royalties, stock options and other financial incentives by Medtronic, a device manufacturer with a $115 Billion market cap. The type of Medtronic hardware which Dr Langston Holly endorses is called "Vertex Max Reconstruction System", which was both Recalled and not FDA approved for posterior cervical spine fusion at the time it was used on this patient and had been recalled several consecutive years thereafter.
Dr Holly uses it to benefit himself financially. Medtronic pays Dr Langston Holly Royalties to teach the UCLA neurosurgery residents.
Furthermore, a Medtronic Sales representative, Brad Schaefer attended the 10 hour surgery and was left alone with the residents as they placed the Medtronic hardware in this patient. Therefore, the residents have practiced on this patient without Dr Holly's constant supervision using this non-FDA approved and Recalled Medtronic device.
Medical records show Dr Holly clocking out of the operating room several times for lengthy periods. Residents cannot operate on patients without supervision. Dr Holly allowed the residents to practice on this patient without supervision, and in fact left alone with Medtronic's Brad Schaefer.
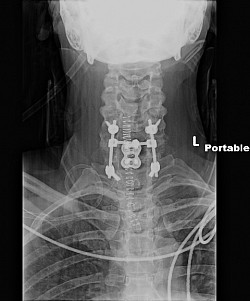
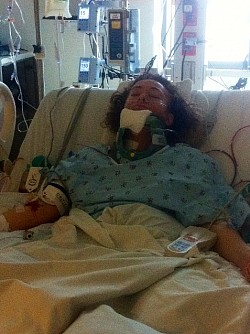
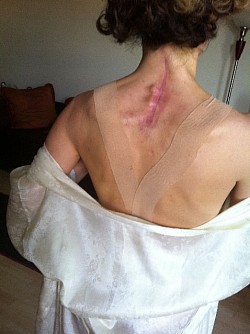
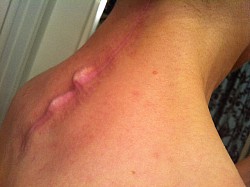
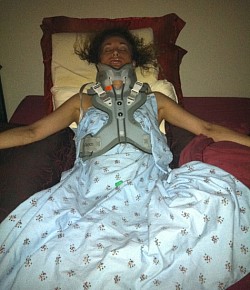
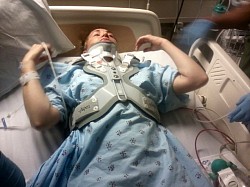
These photos are an example of the Dr Langston Holly spine surgery results. This patient had only one vertebrae fractured after the patient was blind sided in a motor vehicle accident. The patient was neurologically intact without a scratch, just fractures to vertebrae C6.
Without the patient's consent, 2 different spine fusions were performed on her that day. C6 was fused to C7 from the front of the patient's cervical spine. Then Dr Langston Holly had the patient turned over used the Medtronic Vertex Max Reconstructive System titanium in this patient, when it was not medically necessary nor medically indicated.
The patient has discovered Dr Holly's intentions were those of financially benefit for himself and Medtronic; The posterior spine fusion surgery using Medtronic Vertex Max was for Dr Holly's financial benefit.
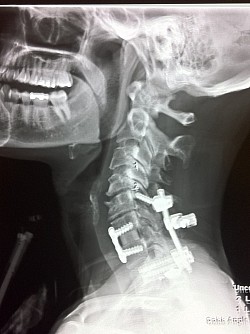
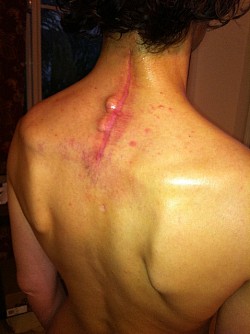
The Medtronic Vertex Max Reconstruction System consists of titanium rods, screws and a bulky CROSSLINK bar. The instructional manual indications state: intended to "fuse the cervical spine to the thoracic spine", from C3-T4, Apparently, this is why the patient's posterior spine was incised to the length of the instructional manual's indications from patients cervical spine to the middle of the patients thoracic spine totaling a 10 inch incision; to accommodate the indication use for the Medtronic Vertex Max Reconstruction System hardware.
In the Medtronic Vertex Max Reconstruction System manual, contraindications loomed large. This patient fell under the list of contraindications. Medtronic Vertex Max Reconstructive System manual specifically says:
- Only patients that meet the criteria described in the indications should be selected.
- Patient conditions and/or pre-dispositions such as those addressed in the aforementioned contraindications should be avoided.
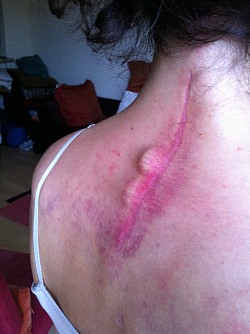
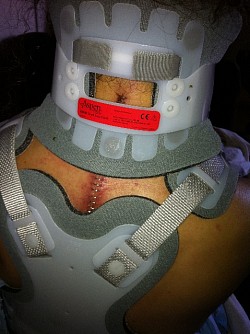
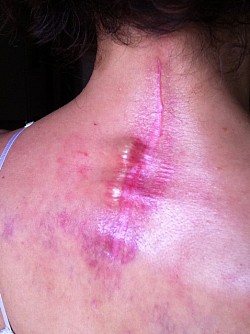
As a result of Dr Holly improperly using this Medtronic device, the patient suffered many of the adverse conditions, including the loose pedicle screws, improper screw placement on the neural foramen and a severe 12inch Fascia Dehiscence. Not only were the posterior muscles and fascia grossly dehisced but the patient was also fused in a straight line (without the crucial cervical lordosis). The combination of the 12 inch fascia dehiscence and fusion deformity caused a compensatory fusion deformity, and subsequent neural compression, subsequent bone spur growth and focal abrasion of the bones above and below the fusion.
Dr Langston Holly failed to diagnose, failed to treat, and failed to refer all these medical complications and injuries to a specialist. Dr Langston Holly forbade the patient to see any other physician at UCLA for all these problems.
Upon second opinions with other Spine surgeons and obtaining CT Scans elsewhere, these second opinion spine surgeons found that the screws were not placed in the bone correctly, and these screws were loose.
The residents supervised by Dr Langston Holly drilled a screw in the patients nerve exit (neural foramen) at a vertebrae that was fused unnecessarily. The screws were not tightened and were loose, clicking and clanking in the patient's spine. It was brought to Dr Langston Holly's attention, but he failed to treat the patient or refer the patient to a specialist. The screw was also causing the patient severe burning pain where the patient was unable to move the left shoulder. Dr Langston Holly Misdiagnosed the frozen shoulder by sending the patient for an MRI which came back as negative.
Dr Langston Holly abandoned care for this patient after the patient notified Dr Holly of the multi-factorial post surgical injuries and complications.
the="" "gold=" " standard"="" for="" spine="" surgery="" is="" "minimal=" " invasive".="" dr="" langston="" holly="" at="" ucla="" medical="" center="" and="" neurosurgery="" department="" performed="" below="" the="" standard="" of="" care.="" dr="" unnecessary="" surgery,="" failed="" to="" treat="" refer="" patient="" a="" specialist="" after="" post="" surgical="" problems="" complications,="" give="" or="" obtain="" informed="" consent="" whatsoever="" from="" patient, injecting="" medications="" too="" quickly,="" improper="" timing="" medication="" administration,="" administering="" much="" one="" time,="" combining="" incompatible="" medications<="" p="">
Since then, the patient has undergone 3 more revision spine surgeries, still not successfully treated due to the difficulty in repairing, where it is impossible to reverse the damage Medtronic and Dr Langston Holly have caused. Furthermore, Medtronic Vertex Max hardware unnecessarily fused additional vertebrae that were not damaged to begin with.
At UCLA spine neurosurgery department, Dr Langston Holly performed this posterior surgery on this patient without any signatures for informed consent from the patient whatsoever, after the UCLA department overdosed the patient with injectable morphine, versed and other sedatives and barbiturates. UCLA over administered Morphine and other sedatives and barbiturates too quickly and over short increments of time to the level where this patient was totally out. It seems illegal for a medical institution and its employees to try to gain consent from a patient who's been completely anesthetized and sedated.
To sum: Dr Langston Holly failed to diagnose, treat and refer this patient to a specialist following his post surgical problems and complications. Dr Langston Holly performed unnecessary surgery without the patient's consent resulting in severe permanent spine deformity. Dr Langston Holly performed open maximal invasive surgery not indicated for the patient. Dr Langston Holly failed to disclose his financial partnership with Medtronic. Dr Langston Holly operated below the standard of care. Medtronic seemingly had Undue Influence over Dr Holly with the financial incentives, stock options and other compensations. The Medtronic Vertex Max Reconstruction System was Recalled and Not FDA approved at the time it was implanted in this patient. The Vertex Max Reconstruction System was Recalled for the same malfunction this patient (and several other reported "Adverse Events") suffered. It seems Medtronic has violated several State and Federal Laws which are supposed to protect patients.
ucla="" over="" administered="" morphine="" and="" other="" sedatives="" barbiturates="" too="" quickly="" in="" small="" time="" increments="" combining="" incompatible="" medications="" rendering="" this="" patient="" completely="" sedated="" overdosed.="" it="" is="" illegal="" to="" try="" gain="" consent="" from="" an="" overdosed="" medicated="" patient. dr="" langston="" holly="" failed="" diagnose,="" treat="" or="" refer="" the="" a="" specialist="" for="" his="" post="" surgical="" problems="" complications,="" dr="" ucla="" medical="" center="" obtain="" informed="" whatsoever="" performing="" 2="" surgeries="" both="" front="" back="" of="" 's="" spine="" totaling="" 10="" hours.="" moreover,="" patient. <="" p="">
ucla="">
the="">